
Biomarker Clinical Phase Outsourcing Services Market Size, Share & Trends Analysis Report By Biomarker Type (Predictive Biomarkers, Surrogate Endpoints), By Therapeutic Area, By End-use, By Region, And Segment Forecasts, 2023 - 2030
- Report ID: GVR-4-68040-138-1
- Number of Pages: 195
- Format: Electronic (PDF)
- Historical Range: 2018 - 2021
- Industry:Healthcare
Market Size & Trends
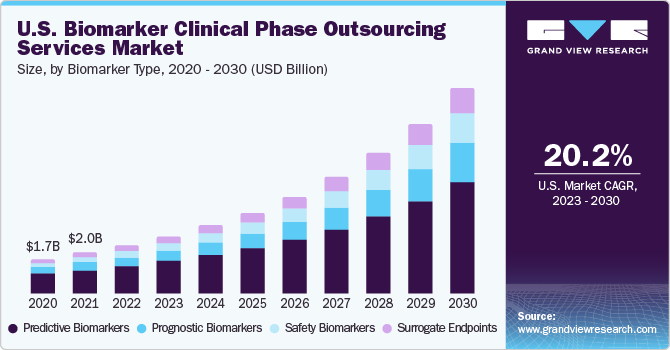
The globalbiomarker clinical phase outsourcing services market size was estimated at USD 6.15 billion in 2022and is expected to grow at a compound annual growth rate (CAGR) of 20.6% from 2023 to 2030. The growing diagnostic applications ofbiomarkers, increase in research and development funding from governments, rapid progress in genomics, proteomics, & imaging, as well as a noticeable shift toward precision medicine are some major factors aiding market growth. Increasing applications of biomarkers is one of the prominent factors augmenting the growth of the market. Biomarkers are measurable indicators, which help in offering information regarding the presence, progression, or severity of a condition or disease.
As a result, the utilization of biomarkers for numerous chronic diseases has witnessed significant growth. For instance, below are a few examples of primary biomarkers that are significantly used in several clinical indications, such as:
Prostate-Specific Antigen (PSA): PSA is a biomarker used in the screening & monitoring of prostate cancer.
CA-125: CA-125 is a biomarker used in the diagnosis and monitoring of ovarian cancer.
HER2/neu: HER2/neu is a biomarker used to identify breast cancer patients who may benefit from targeted therapies.
BNP (B-type natriuretic peptide): BNP is a biomarker used for diagnosing heart failure and assessing its severity.
S100B: S100B is a biomarker utilized in traumatic brain injury (TBI) monitoring and diagnosis.
BRCA1 and BRCA2: Mutations in these genes act as biomarkers in cases of increased risk of ovarian and breast cancers.
Anti-cyclic citrullinated peptide (anti-CCP): Utilized as a biomarker in the condition of rheumatoid arthritis.
These examples highlight the diverse range of biomarkers and their applications in monitoring and diagnosing several disorders. Advancements in biomarker technology are expanding the possibilities for more personalized treatment, accurate and early disease detection, and improved patient outcomes.
The outbreak of the COVID-19 pandemic had a moderate positive impact on the market. The initial phase of the pandemic led to delays in initiating new trials, disruptions in ongoingclinical trialssignificantl以及试验设计中的变化y impacted the demand for biomarker-related services, including data management and sample analysis. Moreover, several pharmaceutical companies and clinical research organizations shifted their priorities toward COVID-19-related clinical trials and research. However, on the brighter side, the outbreak of the COVID-19 infection increased the demand for diagnostic biomarkers to monitor and detect the infection rate, which led to an upsurge in research and development (R&D) activities of the diagnostic assays, thus supporting the revenue revamp in the market. Furthermore, the pandemic enhanced the adoption of data-driven and digital technologies in clinical trials, including biomarker analysis, which has further led to more cost-effective and efficient outsourcing services.
The market witnessed a considerable impact from the geopolitical war between Russia and Ukraine. Pharmaceutical and biopharmaceutical organizations, as well as CROs faced disruptions in drug development processes owing to a reduction in manufacturing activities across war-affected nations by various global pharmaceutical players. Additionally, regulatory changes significantly impact the movement of biomarker products, such as diagnostic devices and assays, across nations, thus delaying vital discovery & development efforts. Geopolitical tensions have driven various changes in import and export policies as well as trade regulations, thus impacting the market to a certain extent.
Biomarker Type Insights
The predictive biomarkers segment is anticipated to witness a lucrative CAGR of 22.5% from 2023 to 2030. The segment’s growth is driven by increasing pipeline products of predictive biomarkers by several pharmaceutical companies. There has been a significant surge across the biopharma and pharmaceutical companies focusing on developing predictive biomarkers for several disease indications, which is anticipated to support the segment’s growth across the analysis period.
For instance, AstraZeneca’s 2023 pipeline includes Potdevin, G; a first assessment of 89-Zr-Crefmirlimab with CD8-PET/CT as a predictive biomarker for response to patients with solid tumors.
Zentalis Pharmaceutical’s 2023 pipeline includes a novel predictive biomarker ZN-c3, in patients with advanced solid tumors.
The surrogate endpoints segment dominated the market with the largest share of 56.0% in 2022. The high share is attributed to the rising investment by contract researchers in developing surrogate endpoints for the diagnosis and monitoring of several chronic ailments. Furthermore, the investment in the development of surrogate endpoint biomarkers is comparatively high, and several clinical research sponsors are putting their best efforts into developing the same. For instance, the development and validation costs of surrogate endpoints are in the range of USD 50 to 115 million, which is significantly higher compared to the other biomarker types. Despite the high prices, the majority of the drug sponsors are continuously making huge investments in the surrogate endpoint development process, thereby supporting its lion’s share.
Therapeutic Area Insights
The oncology segment dominated the market and accounted for the largest revenue share of 34.6% in 2022. High shares of the segment are majorly due to the increasing usage of biomarkers in clinical trials in oncology. There has been a tremendous surge in the total percentage of cancer trials using biomarker analysis for efficient treatment discovery. For instance, in 2022, as per the findings of the evolution of biomarker use in clinical trials for cancer treatments, more than 55% of all cancer trials from 2018 to 2020 involved the use of biomarkers, compared with 15 percent in 2000. Hence, such statistics suggest a robust revenue share for the oncology segment in the market.
The autoimmune diseases segment is anticipated to witness a lucrative CAGR of 22.0% during the analysis period. The high growth of the segment is majorly due to the increasing number of clinical research across diseases associated with autoimmune diseases. For instance, in March 2023, AbbVie Inc. announced positive results from a Phase 2 study of upadacitinib in patients with moderately to severely active systemic lupus erythematosus, a complex autoimmune disorder. Hence, the increase in research and development activities across the condition is one of the major factors supporting the segment’s share.
End-use Insights
The biotechnology companies’ category dominated the market and accounted for the largest revenue share of 41.6% in 2022. This is on account of the increasing number of biopharmaceutical companies that support novel biomarker development for diagnosing and monitoring various chronic ailments. For example, in August 2023, the University of South Australia and Quest Diagnostics announced a collaboration with Envision Sciences for the introduction of a novel biomarker test for prostate cancer. This test aims to assist in detecting patients with particularly aggressive manifestations of the condition.
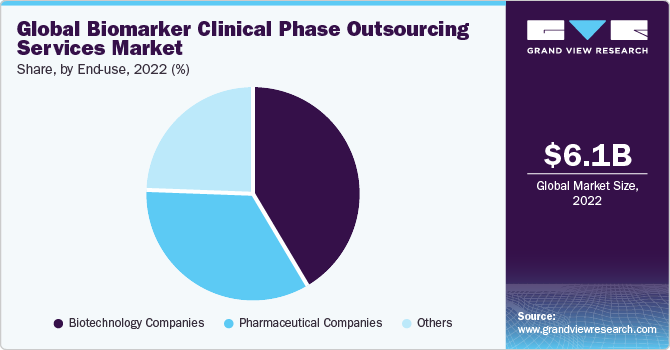
The pharmaceutical companies segment is anticipated to witness a stable CAGR of 20.5% during the forecast timeframe. High growth is primarily due to the increasing number of pharmaceutical companies actively investing in novel drug development that requires biomarker testing. For instance, companies such as Roche; Bristol Myers Squibb; AstraZeneca; Merck; Novartis; and Pfizer are actively investing in biomarker-related drug development trials. Hence, the aforementioned factor is anticipated to support the stable growth of the segment across the analysis period.
Regional Insights
北美最大的收入占share of 42.1% in 2022, owing to the presence of established CROs specializing in biomarker services, such as Laboratory Corporation of America Holdings, ICON plc, Charles River Laboratories, and others. The United States is the leading biomarker outsourcing market, as a large number of pharmaceutical organizations prefer to outsource their manufacturing services to U.S.-based CROs. The preference is aided by the consistent and reliable delivery of premium products and services by CROs based in the country. Furthermore, the U.S. is poised to witness strong demand for biomarker outsourcing services, on account of the growing R&D investments by companies in the life sciences and pharmaceutical sectors.
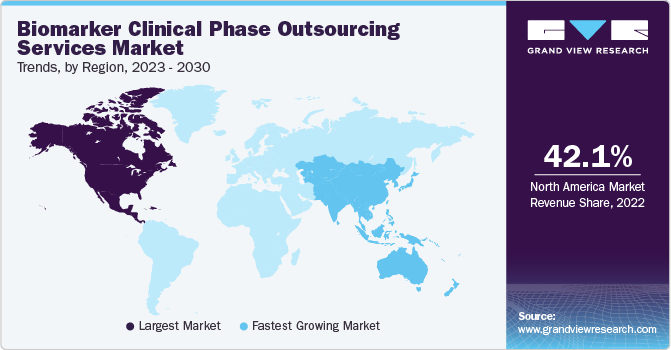
Asia Pacific is anticipated to register the fastest CAGR of 21.4% during the forecast period. Asia Pacific is a highly attractive market for performing clinical trial activities due to supportive regulatory reforms as well as economical clinical research alternatives, especially in India and China. Additionally, presence of U.S. FDA and European Medicines Agency-approved facilities in developing regional economies is poised to drive foreign investments. For instance, Jiangsu, China, is home to approximately 92 contract service organizations, with around 72% of these facilities holding approvals from the EMA and the U.S. FDA. Hence, the aforementioned factors are anticipated to boost the market growth in Asia Pacific.
Key Companies & Market Share Insights
The major players operating across the market are focused on adopting in-organic strategic initiatives such as mergers, partnerships, acquisitions, and others. Moreover, companies are focusing on mergers and partnerships to position themselves in the market better. For instance, in March 2023, Fujirebio Holdings Inc., a contract development and manufacturing organization, and AriBio Co., Ltd. announced a partnership to develop specialized biomarkers for Alzheimer’s disease and other neurodegenerative ailments. Some prominent players in the global biomarker clinical phase outsourcing services market include:
Laboratory Corporation of America Holdings
Parexel International Corporation
ICON plc
Charles River Laboratories
Proteome Sciences
Fujirebio
WuXi AppTec
NorthEast BioAnalytical Laboratories LLC.
Celerion
GenScript ProBio
Biomarker Clinical Phase Outsourcing Services Market Report Scope
Report Attribute |
Details |
Market size value in 2023 |
USD 7.32 billion |
Revenue Forecast in 2030 |
USD 27.17 billion |
Growth rate |
CAGR of 20.6% from 2023 to 2030 |
Base year for estimation |
2022 |
Historical data |
2018 - 2021 |
Forecast period |
2023 - 2030 |
Quantitative units |
Revenue in USD million/billion and CAGR from 2023 to 2030 |
Report Coverage |
Revenue forecast, company share, competitive landscape, growth factors, and trends |
Segments Covered |
Biomarker type, therapeutic area, end-use, region |
Regional scope |
North America; Europe; Asia Pacific; Latin America; Middle East & Africa |
Country scope |
美国;加拿大;U.K.; Germany; France; Italy; Spain; Denmark; Sweden; Norway; China; India; Japan; Australia; Thailand; South Korea; Brazil; Mexico; Argentina; South Africa, Saudi Arabia; UAE; Kuwait |
Key companies profiled |
Laboratory Corporation of America Holdings; Parexel International Corporation; ICON plc; Charles River Laboratories; Proteome Sciences; Fujirebio; WuXi AppTec; NorthEast BioAnalytical Laboratories LLC.; Celerion; GenScript ProBio |
Customization scope |
Free report customization (equivalent up to 8 analysts working days) with purchase. Addition or alteration to country, regional, and segment scope. |
革命制度党cing and purchase options |
Avail customized purchase options to meet your exact research needs.Explore purchase options |
Global Biomarker Clinical Phase Outsourcing Services Market Report Segmentation
This report forecasts revenue growth at global, regional & country levels and provides an analysis of the industry trends in each of the sub-segments from 2018 to 2030. For this study, Grand View Research has segmented the global biomarker clinical phase outsourcing services market report based onbiomarker type, therapeutic area, end-use, and region:
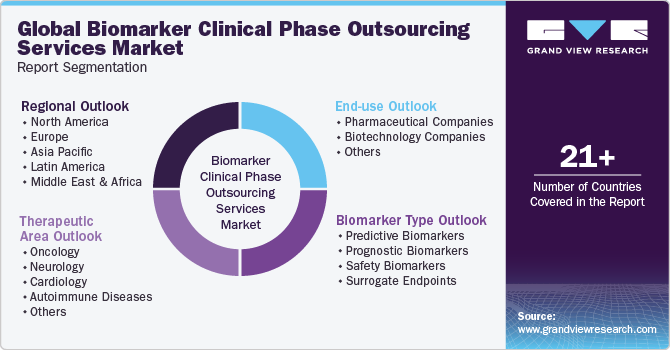
Biomarker Type Outlook (Revenue, USD Million, 2018 - 2030)
Predictive Biomarkers
Prognostic Biomarkers
Safety Biomarkers
Surrogate Endpoints
Therapeutic AreaOutlook (Revenue, USD Million, 2018 - 2030)
Oncology
Neurology
Cardiology
Autoimmune Diseases
Others
End-useOutlook (Revenue, USD Million, 2018 - 2030)
Pharmaceutical Companies
Biotechnology Companies
Others
Regional Outlook (Revenue, USD Million, 2018 - 2030)
North America
U.S.
Canada
Europe
U.K.
Germany
France
Italy
Spain
Denmark
Sweden
Norway
Asia Pacific
Japan
China
India
Australia
South Korea
Thailand
Latin America
Brazil
Mexico
Argentina
Middle East & Africa
South Africa
Saudi Arabia
UAE
Kuwait
Frequently Asked Questions About This Report
b.北美占据的市场份额42.1% in 2022. This is attributable to several pharmaceutical companies preferring to outsource their manufacturing services to CROs based in the U.S. This preference is driven by the consistent delivery of high-quality products and services by U.S.-based CROs.
b.Some prominent biomarker clinical phase outsourcing services market players include Laboratory Corporation of America Holdings, Parexel International Corporation, ICON plc, Charles River Laboratories, Proteome Sciences, Fujirebio, etc.
b.Increasing diagnostic applications of biomarkers, a rise in R&D funding from the government, Rapid advancements in technologies such as genomics, proteomics, and imaging, and the shift toward precision medicine are a few of the factors supporting the market growth.
b.The global biomarker clinical phase outsourcing services market size was valued at USD 6.15 billion in 2022 and is expected to reach USD 7.32 billion in 2023.
b.The global biomarker clinical phase outsourcing services market is expected to grow at a compound annual growth rate of 20.6% from 2023 to 2030 to reach USD 27.17 billion by 2030.
We are committed towards customer satisfaction, and quality service.
"The quality of research they have done for us has been excellent."






