
Biotechnology & Pharmaceutical Services Outsourcing Market Size, Share & Trends Analysis Report By Services (Consulting, Auditing And Assessment, Regulatory Affairs), By End Use (Pharma, Biotech), By Region, And Segment Forecasts, 2022 - 2030
- Report ID: GVR-1-68038-326-3
- Number of Pages: 133
- Format: Electronic (PDF)
- Historical Range: 2018 - 2020
- Industry:Healthcare
Report Overview
The global biotechnology and pharmaceutical services outsourcing market size was valued at USD 66.0 billion in 2021 and is expected to expand at a compound annual growth rate (CAGR) of 5.5% from 2022 to 2030. The COVID-19 pandemic has had a considerable impact on the biotechnology and pharmaceutical services outsourcing industry. Restrictions on movement and stay-at-home orders are helping reduce the spread of COVID-19. However, this is creating an obstacle inclinical trialsand other services intended to find effective treatments for various diseases. The integrity of over 330,000 clinical trials registered on Clinicaltrials.gov remains vulnerable as the novel coronavirus continues to spread worldwide.
As of March 26, 2020, at least 18 pharma or biotech companies reported disturbance in drug development as a result of the pandemic. The shift in focus of biopharmaceutical companies on developing vaccines and therapies in response to COVID-19 has resulted in unintended consequences that may potentially disrupt outsourcing. The recovery from this pandemic has started as a demand for innovative and effective therapies is increasing and driving growth. Rising pricing pressure, lack of internal capabilities, increase in the drug development process cost, and access to the industry expertise are expected to drive the biotechnology and pharmaceutical services outsourcing market during the forecast period. In addition, rising drug development costs coupled with higher failure rates and growing regulatory pressure also have a significant impact on market dynamics, accelerating the growth rate.
Furthermore, well-established Clinical Research Organizations (CROs), regulatory outsourcing firms, management consulting firms, and contract manufacturers are catering to the complex demand of pharmaceutical and biotechnological sectors. Contract research and manufacturing companies are investing in personnel, infrastructure, and technology in order to acquire a significant share of the healthcare outsourcing market revenue. Increasing demand due to the ongoing patent cliff of the biotechnology drugs is anticipated to fuel demand. An increasing number of end-to-end service providers to meet the rising demand for low-cost drug development and manufacturing is further anticipated to propel the growth of the market.
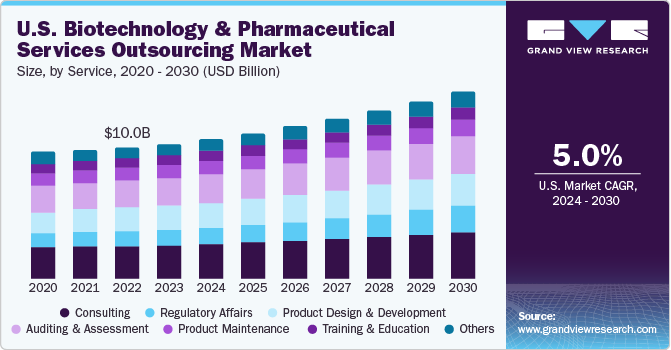
COVID19 impact: Consulting services to generated the highest revenue of 12.0 billion in 2020
Pandemic Impact |
Post COVID Outlook |
During the first quarter of 2020, the impact of the pandemic on the biotech market was relatively mild. The most important indicators - new funds being raised by venture capital (VC) firms, the pace of clinical trials, mergers, and acquisitions, and regulatory approvals (or rejections) - did not change much. In that quarter, there were more biotech IPOs on western exchanges than in the last quarter of 2019. |
In 2021 the biotech sector will have a valuation at or above the valuation it had in late 2019. Pre-clinical work will be robust. Clinical trials will be near the volume of a year earlier. The regulatory review of clinical trials will be more efficient. |
April survey of medical affairs leaders suggests that roughly 95 percent of medical science liaisons (MSLs) globally are engaging physicians entirely remotely, often using virtual engagement platforms rolled out or upgraded in Q1 2020 to quickly support new ways of working. |
新的生物技术公司预计提高娘家姓的ded capital. There will be an uptick in M&A activity as large pharmaceutical companies acquire smaller companies (see, for example, Alexion’s acquisition of Portola at a valuation much below that company’s value in mid-2019) |
Due to the recent upheaval caused by the COVID-19 pandemic, the U.S. Food and Drug Administration (FDA) has issued guidance providing some flexibility for clinical trials, however, there is no one-size-fits-all approach to applying such guidance. |
Biopharmaceutical Innovators continues to Lead the Charge in Fight Against Coronavirus. |
Moreover, novel drug delivery mechanisms and new product launches are anticipated to drive the outsourcing demand. Many companies are opting for outsourcing owing to increasing competition in the healthcare industry. For instance, in August 2020, Pfizer Inc. signed a three-year agreement with PPD, Inc. to provide drug development services. Technological advancements and focus on customized care shorten a product’s life cycle, which leads to the rapid development of new products. Increasing R&D activities for new drug development, combination products, and other advanced medicines have increased the demand for contract Biotechnology and Pharmaceutical services.
目前,全球制药行业the second-highest R&D intensity measures of any sector, which indicates that the spending on R&D is increasing and the overall R&D spending is likely to grow during the forecast period. According to Evaluate Pharma in 2019, the pharmaceutical R&D spend was about USD 179 billion in 2018 and is expected to exhibit a CAGR of 3.0% between 2018 and 2024. Growing R&D on large molecules has increased the demand for contract services. Thus, increasing R&D spending is expected to drive in particular the biotechnology services outsourcing market during forecast period.
Service Insights
The consulting services segment dominated the market and accounted for the largest revenue share of 19.5% in 2021. The segment is expected to maintain its position over the forecast period. This can be attributed to the increasing M&A activities and constantly changing regulatory protocols. However, pharma and biotech market have witnessed the continuous entry of new players such as Signa Medical Writing, bioSyntagma, and Fieve Clinical Research, Inc. These players need to be compliant with set standards and norms, for which consulting is essential as these new entrant’s lack in such capabilities.
However, the other services segment was valued at USD 10,649.1 million in 2021 and is expected to witness a higher CAGR of 6.4% over the forecast period owing to rising outsourcing of generics and biosimilar manufacturing as CMOs are offering services at lower cost. Other segment is inclusive of contract manufacturing, product upgrade and IT consulting. Biostatistics outsourcing is increasing owing to growing concerns about biotechnology and pharmaceutical companies with regard to competition in product commercialization, clinical trial complexity, and the quality of clinical trials that have boosted the importance of biostatistics.
End-use Insights
The pharma segment dominated the market and accounted for the largest revenue share of 55.7% in 2021. The segment is expected to register a CAGR of 5.7% over the forecast period. An increase in R&D spending by pharmaceutical companies for the development of potential novel products and a rise in investments by CROs for the development of core capabilities are expected to drive the market in the region in the coming years. Contract service providers are recognized as an effective strategic decision to curb the issues of drug shortfall and high production costs, as well as meet the growing demand.
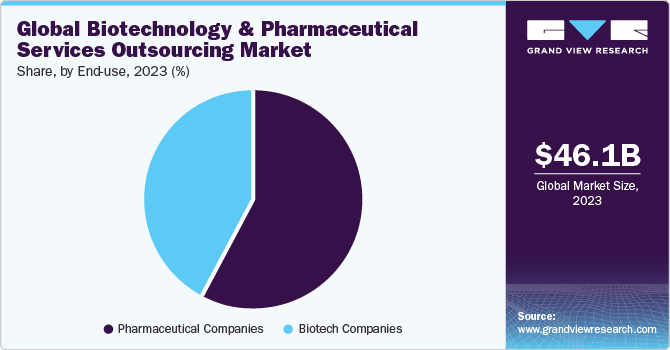
Lack of resources has prompted many pharmaceutical companies to outsource drug development and manufacturing of their products to CROs and CMOs. The CROs and CMOs offer highly sophisticated development and manufacturing services, such as preclinical development, clinical development, commercial manufacturing, clinical manufacturing, precision injection molding, high-speed automation, assembly, fill/finish, and others for a range of pharma products.
Regional Insights
North America dominated the market and accounted for the largest revenue share of 53.8% in 2021. This growth is owing to the presence of several established CROs and CMOs such as Covance Inc., IQVIA, Catalent, and Samsung Biologics, and growing R&D investments by life sciences and pharmaceutical companies in the region. The presence of stringent regulatory policies and increase in R&D expenditure are among the factors likely to boost the demand for outsourcing of services by pharmaceutical and biotechnology companies in the region. In addition, the expansion of foreign CMOs and CROs into the country is expected to propel market growth. For instance, in September 2019, Bora Pharmaceuticals received FDA approval for its CMO services and in October 2019, it opened a sales office in Delaware to expand its presence outside Asia and strengthen its market position.
Asia Pacific biotechnology and pharmaceutical services outsourcing market is expected to witness a CAGR of 6.1% over the forecast period owing to an increase in investments by developed countries and various regulatory reforms in clinical trial evaluation to align with standards of various countries investing in the region. Low cost of drug development and manufacturing, and availability of skilled workforce are likely to foster contract development and manufacturing in this region. Moreover, economic policy reforms in countries such as China are anticipated to create an open and balanced economy, which presents ample opportunity for market players to invest in this region. In July 2017, Novotech expanded its site management services in Korea with the launch of Novotech SMO Korea Co. Limited.
Key Companies & Market Share Insights
The market is very competitive. Some of the players operating in this market are The Quantic Group, IQVIA, Parexel International Corporation, Charles River Laboratories. The main factor affecting this competitive nature are quick adoption of merger and acquisition, collaboration, regional expansion, service portfolio expansion, and competitive pricing as the key strategies to sustain in the highly competitive environment and acquire a higher market share. For instance, Parexel International Corporation formed a strategic partnership with Veeva in April 2021 to accelerate clinical trials by leveraging technology and process innovation. The collaboration can help improve study efficiency and accelerate the delivery of new therapies to patients. Some of the prominent players in the biotechnology and pharmaceutical services outsourcing market include:
Parexel International Corporation
The Quantic Group
IQVIA
Lachman Consultant Services, Inc.
GMP Pharmaceuticals Pty Ltd.
Concept Heidelberg GmbH
Covance Inc.
Charles River Laboratories
PRA Health Sciences
ICON plc
Biotechnology & Pharmaceutical Services Outsourcing Market Report Scope
Report Attribute |
Details |
Market size value in 2022 |
USD 70.5 billion |
Revenue forecast in 2030 |
USD 108.0 billion |
Growth rate |
CAGR of 5.5% from 2022 to 2030 |
Base year for estimation |
2021 |
Historical data |
2018 - 2020 |
Forecast period |
2022 - 2030 |
Quantitative units |
Revenue in USD million and CAGR from 2022 to 2030 |
Report coverage |
Revenue forecast, company ranking, competitive landscape, growth factors, and trends |
Segments covered |
Service, end-use, region |
Regional scope |
北美;欧洲;亚太地区;拉丁美洲; MEA |
Country scope |
U.S.; Canada; U.K.; Germany; France; Italy; Spain; Australia; South Korea; China; India; Japan; Brazil; Mexico; Argentina; South Africa; Saudi Arabia; UAE |
Key companies profiled |
The Quantic Group; IQVIA; Parexel International Corporation; Lachman Consultant Services, Inc.; GMP Pharmaceuticals Pty Ltd.; Concept Heidelberg GmbH; Covance, Inc.; Charles River Laboratories; PRA Health Sciences; ICON plc |
Customization scope |
Free report customization (equivalent up to 8 analysts working days) with purchase. Addition or alteration to country, and segment scope. |
Pricing and purchase options |
Avail customized purchase options to meet your exact research needs.Explore purchase options |
Segments Covered in the Report
This report forecasts revenue growth at the global, regional, and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2018 to 2030. For the purpose of this study, Grand View Research has segmented the global biotechnology and pharmaceutical services outsourcing market report on the basis of service, end-use, and region:
Service Outlook (Revenue, USD Million, 2018 - 2030)
Consulting
Regulatory compliance
Remediation
Quality management systems consulting
Others
Auditing and assessment
Regulatory affairs
Clinical trial applications & product registration
Regulatory writing & publishing
Legal representation
Others
Product maintenance
Product design & development
Product testing & validation
Training & education
Others
End-use Outlook (Revenue, USD Million, 2018 - 2030)
Pharma
Biotech
Regional Outlook (Revenue, USD Million, 2018 - 2030)
North America
U.S.
Canada
Europe
U.K.
Germany
France
Italy
Spain
Asia Pacific
India
China
Japan
Australia
South Korea
拉丁美洲
Brazil
Mexico
Argentina
Middle East & Africa
South Africa
Saudi Arabia
UAE
Frequently Asked Questions About This Report
b.Key factors that are driving the biotechnology & pharmaceutical services outsourcing market growth include growing drug development costs coupled with high clinical development failure rates and increasing competition within the healthcare drug manufacturers.
b.The global biotechnology & pharmaceutical services outsourcing market size was estimated at USD 66.0 billion in 2021 and is expected to reach USD 70.5 billion in 2022.
b.The global biotechnology & pharmaceutical services outsourcing market is expected to grow at a compound annual growth rate of 5.5% from 2022 to 2030 to reach USD 108.0 billion by 2030.
b.North America dominated the biotechnology & pharmaceutical services outsourcing market with a share of 54.0% in 2021. This is attributable to the presence of several multinational and local consulting firms in this region.
b.Some key players operating in the biotechnology & pharmaceutical services outsourcing market include Catalent, Pharmaceutical Product Development LLC, AbbVie, Baxter BioPharma Solutions, Patheon, Grifols International, S.A., Dalton Pharma Services, and Boehringer Ingelheim Biopharmaceuticals GmBh, and Lonza AG.





