
HPV Testing And Pap Test Market Size, Share & Trends Analysis Report By Test Type, By Application (Cervical Cancer Screening, Vaginal Cancer Screening), By Product, By Technology, By End Use, By Region, And Segment Forecasts, 2021 - 2028
- Report ID: 978-1-68038-895-4
- Number of Pages: 120
- Format: Electronic (PDF)
- Historical Range: 2016 - 2019
- Industry:Healthcare
Report Overview
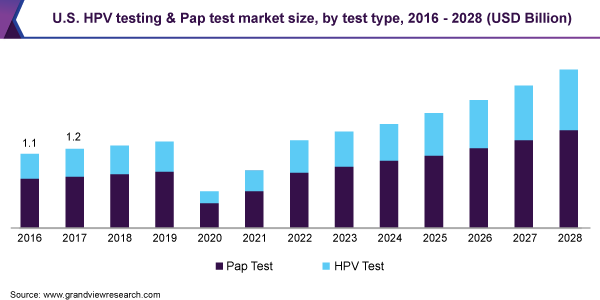
The global HPV testing and Pap test market size was valued at USD 1.7 billion in 2020 and is expected to expand at a compound annual growth rate (CAGR) of 16.4% from 2021 to 2028. Technological advancements, increasing number of cervical cancer screening programs, and high incidence of cervical cancer are some major factors driving the market for HPV testing and Pap test. However, the outbreak of Covid-19 and the implementation of restrictions to stay-at-home across the globe have rigorously reduced routine cancer screenings.
The decline in screenings was steep and swift; as of April 25, 2020, the decline recorded for breast and cervical cancer screening was 94%. During the lockdown, screening of cervical cancer decreased to 80% relative to the baseline, among the 1.5 million population of women is the Kaiser Permanente Southern California (KPSC) network. Segmenting the screening as per age, for women aged between 21 and 29, screening rates of cervical cytology per 100 individual months declined 78%. For women aged between 30 and 65, HPV test screening rates declined 82% per 100 person-months.
宫颈癌是最常见的癌症之一mong women above the age of 40. According to estimates from the WHO, cervical cancer is the fourth most frequent cancer, with approximately 570,000 cases and over 311,000 deaths in 2018. It is estimated that approximately 99% of cervical cancer cases are typically linked with HPV infections. Cervical cancer is mostly diagnosed among women aged between 35 and 44 with the average rate of diagnosis being at 50 years. According to data from the U.S. Department of Health and Human Services, the rate of new cervical cancer cases was estimated to be around 7.4 per 100,000 women, with the death rate being 2.2 per 100,000 women each year.
Growing demand for technologically advanced diagnostic procedures for screening cervical and vaginal cancers is one of the crucial factors expected to drive the market for HPV testing and Pap test over the forecast period. Various technological advancements, such as the development of HPV-type 16 E7-specific human immunologic assays used in non-HLA2 types, cobas system approvals, and usage of various molecular markers in screening procedures, are expected to drive the market for HPV testing and Pap test. In 2019, researchers at Massachusetts General Hospital's Center for Systems Biology and Harvard Medical School, Boston presented a novel screening handheld device that usesArtificial Intelligence(AI) for HPV detection. The novel device detects the presence of HPV strains 16 and 18 in a turnaround time of 2 hours. Similarly, researchers at Pathogen Discovery Laboratory have developed a diagnostic device that detects the presence of precancerous markers in HPV diagnosis.
此外,越来越多的意识在总结ms by various organizations, such as the National Cervical Cancer Coalition, WHO, CDC, and the U.S. Preventive Services Task Force (USPSTF), for cervical cancer screening is one of the major factors expected to drive the market for HPV testing and Pap test during the forecast period. In March 2019, the U.K. government rolled out its first national cervical screening campaign called Cervical Screening Saves Lives. The campaign run by Public Health England (PHE) was initiated owing to a reduced number of screenings by women in the U.K. The campaign was used to facilitate increased awareness about the disease and initiate screening of the disease.
Test Type Insights
The Pap test segment dominated the market for HPV testing and Pap test in 2020 with a revenue share of 64.0%, owing to its widespread implementation in screening programs. Furthermore, increasing initiatives to increase awareness about Pap tests is further expected to fuel the segment growth. In March 2019, Public Health England launched the first government campaign to create awareness about cervical cancer screening using smear tests. Also, in 2017, “Smear your Mea” was founded, which is a community funded by the New Zealand Health campaign. It encourages women to get smear tests done and prevents the occurrence of cervical cancer.
The HPV testing segment is anticipated to witness a CAGR of 17.6% over the forecast period, owing to the commercialization of innovative tests and increasing government recommendations to use more efficient HPV screenings. In July 2018, the Society of Obstetricians and Gynecologists of Canada stated that Pap tests should be replaced by tests that detect high-risk HPVs. HPV tests are DNA-based and enable the detection of specific strains of the virus in the specimen. HPV tests during initial screening help in the detection of abnormal cells that may become cancerous. This helps in the early prevention of disease, allowing healthcare professionals to opt for various treatment and/or preventive options.
Application Insights
The cervical cancer screening segment dominated the market for HPV testing and Pap test and held the largest revenue share at 75.7% in 2020. The segment is expected to witness a CAGR of 16.9% during the forecast period. This dominance and fast growth are anticipated to the higher incidence of cervical cancer as compared to vaginal cancer. In addition, continuous public-private initiatives to increase cervical cancer screening rates are also expected to support the segment growth. In 2018, the WHO launched the Plan of Action for Cervical Cancer Prevention and Control 2018-2030. This program aims to improve screening of the disease and timely treatment through innovative strategies.
On the other hand, the vaginal cancer screening segment is expected to witness a CAGR of 14.4% during the forecast period. Increasing prevalence and high mortality rates of vaginal cancer have propelled the demand for effective screening tests. According to a 2018 report by the American Cancer Society, an estimated 1 out of every 1,100 women is likely to develop vaginal cancer. In 2018, 5,350 women were diagnosed with vaginal cancer in the U.S. The number of vaginal cancer-related deaths in the U.S. was 1,430 in the same year.
Product Insights
The consumables segment dominated the market for HPV testing and Pap test and accounted for the largest revenue share of 65.7% in 2020, owing to its repetitive usage in HPV and cervical screenings. Also, continuous development activities by key players operating in the market and the introduction of innovative consumables, such as assays & kits, for more accurate diagnosis of HPV and Pap are anticipated to boost segment growth. For instance, in September 2020, Becton, Dickinson and Company submitted a Premarket Approval application to the FDA to approve the usage of Thin Prep Pap Test PreservCyt Solution vial along with its On clarity HPV Assay. With this PMA, the company aims to expand the usage of its On clarity HPV Assay in the U.S. and improve patient management.
The services segment is expected to witness the fastest CAGR during the forecast period, owing to the introduction of self and at-home HPV screening services to increase the frequency of cervical cancer screening rates in key markets. In January 2019, Nurx, a U.S.-based telemedicine company, introduced its Home HPV Screening test, which enables women to identify their risk affordably and conveniently for cervical cancer from home in the U.S.
Technology Insights
The other technologies segment dominated the market for HPV testing and Pap test and accounted for the largest revenue share of 47.9% in 2020. This large share can be attributed to the high adoption of colposcopy and cystoscopy techniques in HPV and cervical cancer screenings. The other technologies segment includes colposcopy and cystoscopy.
另一方面,PCR段预计witness the fastest growth in the technology segment, owing to the implementation of new guidelines to utilize DNA HPV testing methods due to its better accuracy and cost-effectiveness. The use of PCR for HPV testing improves the sensitivity of cervical cancer screening programs by early identification of risky lesions in women aged 30 and older with normal cytology. It also reduces the requirement for unneeded colposcopy and treatment in women aged 21 and older with cytology results displaying atypical squamous cells of undetermined significance.
End-use Insights
The hospitals and clinics segment dominated the market for HPV testing and Pap test and accounted for the largest revenue share of 44.5% in 2020, as they serve as primary care settings for diagnosis and treatment of all diseases, including HPV infections and cervical cancer. Furthermore, the rise in healthcare expenditure globally and continuous changes in the healthcare industry have led to an increase in the need for hospitals and clinics with enhanced diagnostic services. Thus, several hospitals and clinics in key markets are adopting technologically advanced solutions to provide more accurate and efficient results. For instance, in March 2020, India-based Yashoda Hospitals launched an FDA-approved HPV DNA test to improve the early detection and prevention of cervical cancer in women in India.
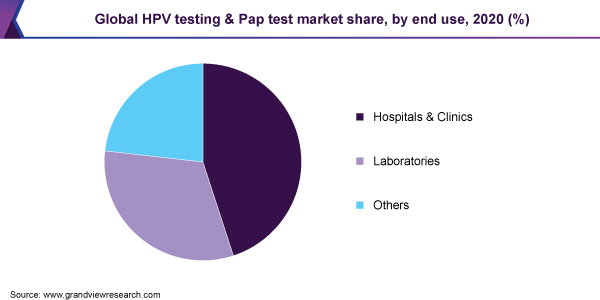
The other segment is expected to show the fastest growth in the end-use segment, owing to the increasing use of the POC tests in-home care settings. Recent advancements in the POC test kits have resulted in shorter turnaround times and error-free results, which is expected to boost the market for HPV testing and Pap test over the forecast years. However, the lack of availability of highly accurate POC testing kits is expected to be a significant factor hampering its market growth during the study period. Furthermore, the growing adoption of self-sampling for cervical cancer screening is also anticipated to significantly support the growth. Self-sampling of HPV has the potential to overcome various identified barriers, such as cultural and community.
Regional Insights
North America dominated the HPV testing and Pap test market and held the largest revenue share of 41.1% in 2020 and is projected to maintain the lead throughout the forecast period. High awareness about early diagnosis of cervical cancer, well-established screening guidelines, and favorable healthcare reimbursement scenario are the factors driving the growth of the market in the region. In addition, high affordability, adoption, and access to technologically advanced products and services have further boosted the growth of the market in the region. Furthermore, the local presence of leading players and ongoing strategic initiatives undertaken by them has significantly supported the growth of the market in the region. For instance, Abbott Laboratories; Becton, Dickinson and Company; Quest Diagnostics; Hologic, Inc. Femasys, Inc.; Arbor Vita Corporation are some HPV and Pap test providers with headquarters in the U.S.
In Asia Pacific, the market for HPV testing and Pap tests is expected to exhibit an exponential CAGR during the forecast period. This high growth can be attributed to the high prevalence of HPV and cervical cancer in key countries, such as Japan, China, and India. In addition, technological advancements in the Asia Pacific region are also anticipated to fuel the market growth during the forecast period. For instance, in January 2018, Seegene, Inc. utilized a new artificial intelligence-based assay development system to simultaneously detect around eight, various DNA targets for each sexually transmitted infection as well as meningitis. Moreover, strategic initiatives undertaken by public-private organizations to improve cervical cancer screening in this region are one of the major factors expected to aid growth during the forecast period.
Key Companies & Market Share Insights
Key players are engaged in organic and inorganic growth strategies such as new product development, mergers and acquisitions, and geographical expansion to capture larger shares in the market. For instance, In November 2018, Qiagen N.V. introduced the QIAscreen HPV PCR Test - a molecular diagnostic test used to diagnose 15 high-risk genotypes of HPV. The assay includes genotyping functionality and screening of HPV. Some of the prominent players in the global HPV testing and Pap test market include:
Abbott Laboratories
Qiagen N.V.
Becton, Dickinson and Company
Quest Diagnostics, Inc.
Hologic, Inc.
F。Hoffmann-La Roche
Femasys, Inc.
Arbor Vita Corporation
NURX, Inc.
Seegene, Inc.
Thermo Fisher Scientific, Inc.
bioMérieux SA
HPV Testing And Pap Test MarketReport Scope
Report Attribute |
Details |
Market size value in 2021 |
USD 2.5 billion |
Revenue forecast in 2028 |
USD 7.3 billion |
Growth Rate |
CAGR of 16.4% from 2021 to 2028 |
Base year for estimation |
2020 |
Historical data |
2016 - 2019 |
Forecast period |
2021 - 2028 |
Quantitative units |
Revenue in USD million and CAGR from 2021 to 2028 |
Report coverage |
Revenue forecast, company ranking, competitive landscape, growth factors, and trends |
Segments covered |
Test type, application, product, technology, end-use, region |
Regional scope |
North America; Europe; Asia Pacific; Latin America; MEA |
Country scope |
U.S.; Canada; Germany; U.K.; France; Italy; Spain; Russia; Japan; China; India; South Korea; Australia; Singapore; Brazil; Mexico; Argentina; South Africa; Saudi Arabia; UAE |
Key companies profiled |
Abbott Laboratories; Qiagen N.V.; Becton, Dickinson and Company; Quest Diagnostics, Inc.; Hologic, Inc.; F. Hoffmann-La Roche; Femasys, Inc.; Arbor Vita Corporation; NURX, Inc.; Seegene, Inc.; Thermo Fisher Scientific, Inc.; bioMérieux SA |
Customization scope |
Free report customization (equivalent up to 8 analyst’s working days) with purchase. Addition or alteration to country, regional & segment scope. |
Pricing and purchase options |
Avail customized purchase options to meet your exact research needs.Explore purchase options |
Segments Covered in the Report
This report forecasts revenue growth at global, regional, and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2016 to 2028. For the purpose of this study, Grand View Research has segmented the global HPV testing and Pap test market report on the basis of test type, application, product, technology, end-use, and region:
Test Type Outlook (Revenue, USD Million, 2016 - 2028)
HPV Testing
巴氏早期癌变探查试验
Application Outlook (Revenue, USD Million, 2016 - 2028)
Cervical Cancer Screening
Vaginal Cancer Screening
Product Outlook (Revenue, USD Million, 2016 - 2028)
Instruments
Consumables
Services
Technology Outlook (Revenue, USD Million, 2016 - 2028)
PCR
Immunodiagnostics
Other Technologies
End-use Outlook (Revenue, USD Million, 2016 - 2028)
Hospitals & Clinics
Laboratories
Other End Users
Regional Outlook (Revenue, USD Million, 2016 - 2028)
North America
U.S.
Canada
Europe
Germany
U.K.
France
Italy
Spain
Russia
Asia Pacific
Japan
China
India
South Korea
Australia
Singapore
Latin America
Brazil
Mexico
Argentina
MEA
South Africa
Saudi Arabia
UAE
Frequently Asked Questions About This Report
b.Key factors that are driving the HPV testing & Pap test market growth include increasing incidence rate of cervical and vaginal cancer and rising awareness created by various government organizations in order to increase cervical cancer screening.
b.The global HPV testing & Pap test market size was estimated at USD 1.7 billion in 2020 and is expected to reach USD 2.5 billion in 2021.
b.The global HPV testing & Pap test market is expected to grow at a compound annual growth rate of 16.4% from 2021 to 2028 to reach USD 7.3 billion by 2028.
b.North America dominated the HPV testing & Pap test market with a share of 41.23% in 2019. This is attributable to increased cases of cervical cancer and the growing awareness amongst women due to the various government initiatives and research studies that were undertaken in this direction.
b.Some key players in the HPV testing & Pap test market include Abbott Laboratories, Qiagen N. V., Becton, Dickinson and Company, Quest Diagnostics, Hologic Inc., Roche, Arbor Vita Corporation, Femasys Inc., Onco Health Corporation, and Seegene Inc.
b.The Pap test segment dominated the market for HPV testing & Pap test in 2020 with a revenue share of 64.0%, owing to its widespread implementation in screening programs.
b.The cervical cancer screening segment dominated the market for HPV testing & Pap test and held the largest revenue share at 75.7% in 2020.
b.The consumables segment dominated the market for HPV testing & Pap test and accounted for the largest revenue share of 65.7% in 2020, owing to its repetitive usage in HPV and cervical screenings.
b.The other technologies segment (includes colposcopy and cystoscopy), dominated the market for HPV testing and Pap test and accounted for the largest revenue share of 47.9% in 2020.
b.The hospitals and clinics segment dominated the market for HPV testing & Pap test and accounted for the largest revenue share of 44.5% in 2020, as they serve as primary care settings for diagnosis and treatment of all diseases, including HPV infections and cervical cancer.




